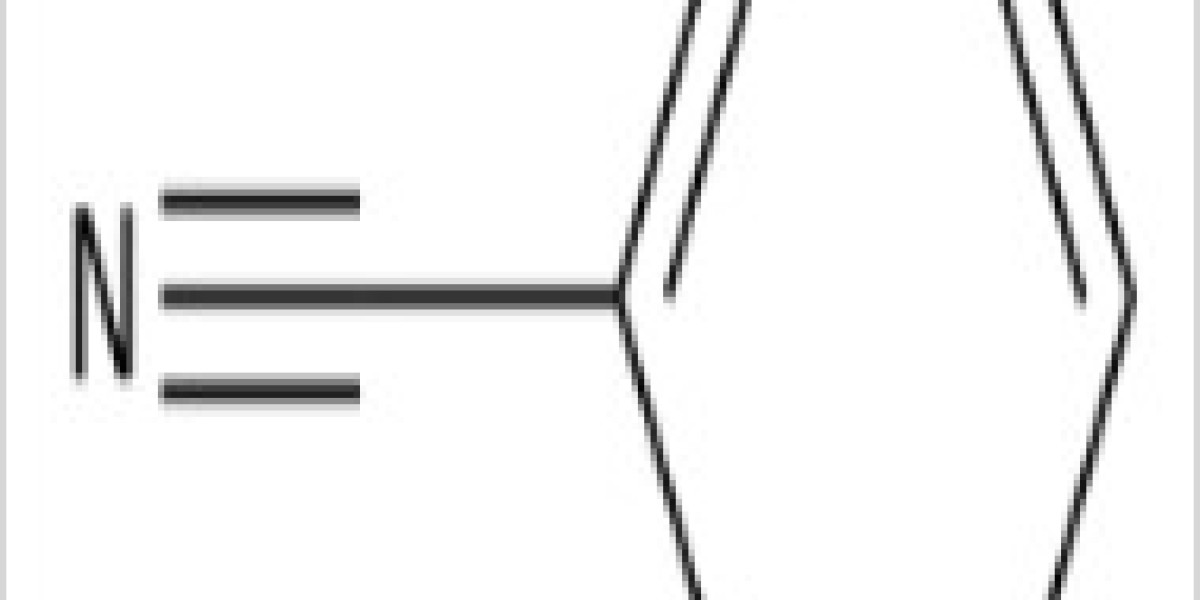
Benzonitrile is the chemical compound with the formula C 6H 5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.
Benzonitrile is a stable compound to pyrolysis, and its decomposition starts above 550°C with a very low decomposition rate. A study performed in a flow reactor on N2 saturated with benzonitrile [8] in the temperature range 550–600°C showed that the main pyrolysis products of this compound are HCN, benzene, monocyanodiphenyls, dicyanodiphenyls, and dicyanobenzenes as well as char. The reaction takes place by a radicalic mechanism, starting with the following initiation reaction:
Benzonitriles and phenoxy acids are widely applied as salts or esters, but they are hydrolysed to their respective phenols or acids in the matrix. Extraction of residues from soil and water is commonly performed at acidic pH with organic solvents of medium polarity. The extraction of these herbicides from vegetable matter is often done with aqueous solutions at basic pH, followed by extraction with organic solvents.
Purification of extracts is required in most cases and this step is accomplished by liquid–liquid partition at basic pH or by chromatography on silica columns.
Benzonitriles and Phenoxy Acids
Benzonitriles and phenoxy acids are widely applied as salts or esters, but they are hydrolyzed to their respective phenols or acids in the matrix. Extraction of residues from soil and water is commonly performed at acidic pH with organic solvents of medium polarity. Initially, the extraction of these herbicides from vegetable matter was done with aqueous solutions at basic pH, followed by extraction with organic solvents.At present, different techniques such as SPME for aqueous samples, UAE for soil and MAE or PLE for plant samples are often used. QuEChERS is also used for the extraction of acidic herbicides from soil and plant samples after alkaline hydrolysis with sodium hydroxide.
Shi and colleagues reported using benzonitriles as the substrates to produce the same secondary amine products with RuCl3/PPh3/K2CO3 as catalyst at 140°C6 (Scheme 7.8). In this reaction, secondary amines were normally synthesized with up to 95% yield. However, N,N,N-diethyl-benzyl amine was produced with 90% yield if ethanol was applied.
Benzonitrile, the simplest cyanoaromatic, is a clear liquid with an almond-like odor. Methods for synthesizing it include
heating sodium benzenesulfonate with NaCN,
adding benzenediazonium chloride to a hot aqueous solution of NaCN and CuSO4,
oxidizing toluene in the presence of ammonia,
dehydrating benzamide, and
treating bromobenzene with CuCN or NaCN.
Despite these many synthetic methods, the only uses for benzonitrile are as a solvent or a precursor to benzoguanamine, a derivative of melamine. But recently, the molecule was the subject of a “stellar” discovery.
Brett A. McGuire, at the National Radio Astronomy Observatory in Charlottesville, VA, and collaborators identified benzonitrile in interstellar dust. They estimate that the dust cloud is 430 light-years away. This is the first aromatic molecule to be discovered in space, even though astrochemists estimate that 10% of all interstellar carbon consist of polynuclear aromatics.