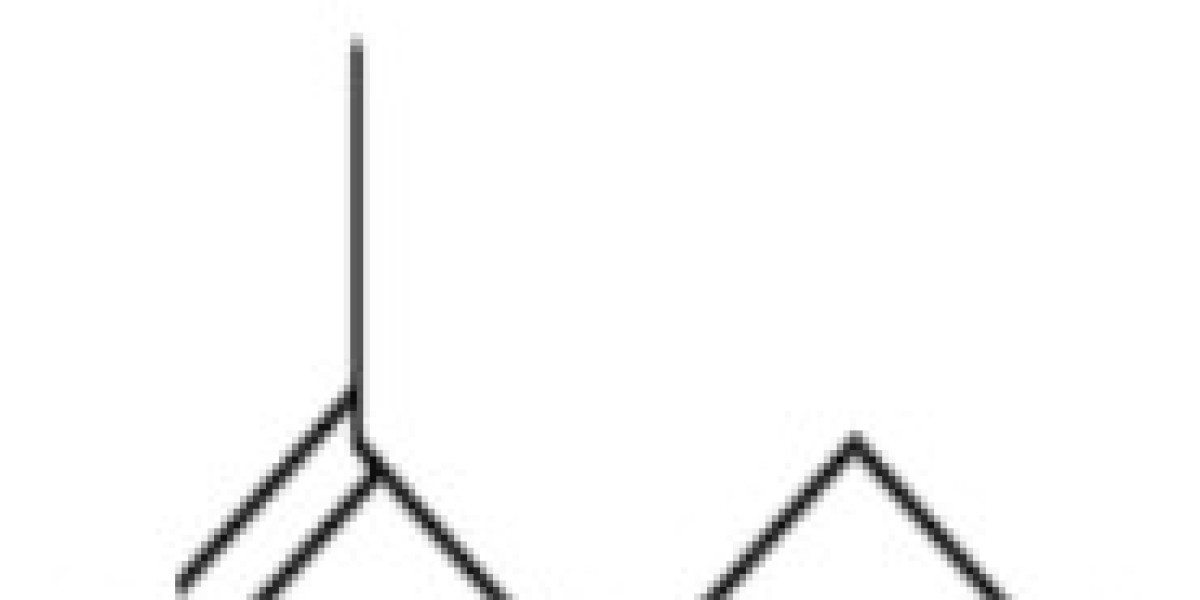
What is the Formula for Ethyl Acetate boiling point?
The formula for ethyl acetate boiling point is CH3COOCH2CH3.
Ethyl acetate boiling point formula is given here along with its structure. Ethyl acetate, also known as ethyl ethanoate is one organic compound and is mainly used as a solvent in different reactions. Ethyl acetate boiling point is obtained from the direct esterification of ethyl alcohol with acetic acid, a process which involves mixing acetic acid with an excess of ethyl alcohol and adding a small amount of sulphuric acid.
The chemical and structural formula of ethyl ethanoate is mentioned below.
Structure & Formula for Ethyl Acetate boiling point
Ethyl ethanoate is generally abbreviated as EtOAc, EA, or ETAC. It has a molar mass of 88.106 g/mole. The carbonyl group carbon has a sp2 hybridization and the other part of the molecule has a tetrahedral geometry. The chemical formula for ethyl acetate boiling point and its structural formula are given below.
Ethyl acetate boiling point is highly flammable and generally found in alcoholic drinks like wines. It is also used as an organic solvent in paints, cellulose, rubber, etc.
Ethyl Acetate boiling point Properties
Commercially, ethyl acetate is made from 10% acetic acid “quick vinegar” process and 50% alcohol “high wines” by a distillation process. A ternary mixture of ethyl acetate, water and ethyl alcohol distils first. Ethyl acetate boiling point is a colourless and sweet-smelling ester of acetic acid and ethanol.
Ethyl Acetate Uses
Used as an indirect food additive in certain packaging materials.
Used as an oxygenated solvent widely used in the inks, pharmaceuticals and fragrance sectors.
Used as flavouring agents and preservatives in the food industry.
Used as a good solvent for nitrocellulose, fats, varnishes, lacquers inks and aeroplane dopes, it is used in the production of smokeless powder, artificial leather, photographic films and plates and artificial silk.
Used as a cleaning agent in the textile industry.
Health Hazard
Acute toxicity was reported while the inhalation in rats as 5.6g/kg after 8hrs.
It is tested in petroleum produced no irritation after 48hr closed patch test in 25 human subjects.
It is tested at a concentration of 10% in petroleum and produced no sensitization reactions.
It is hydrolysed to ethyl alcohol which is then partly excreted in the expired air and urine.
Ethyl acetate boiling point is used as a solvent for varnishes, lacquers, dry cleaning, stains, fats and nitrocellulose. It is released during the production of artificial silk and leather, and during the preparation of photographic films and plates. It is released during the manufacture of linoleum, and 'plastic' wood, dyes, pharmaceuticals, drug intermediates, acetic acid, artificial fruit flavorings and essences, and perfumes and fragrances. Ethyl acetate boiling point is used as a solvent in nail polish, nail polish remover, base coats and other manicuring products. Ethyl acetate boiling point is present in wines.
Ethyl acetate is a flammable liquid, and an explosion hazard. It is slightly soluble in water, but soluble in most organic solvents.
Ethyl acetate boiling point will enter the body if we breathe in contaminated air, or eat or drink contaminated materials. Ethyl acetate can pass through the skin.