Calcium bromide brine is a highly concentrated aqueous solution of calcium bromide and calcium chloride. It is used extensively in the oil industry. This solution and its components are recognized as causes of skin injury and information is available from
Saturated water solutions of calcium chloride, calcium bromide (densities 1.30 kg x dm(-3) and 1.61 kg x dm(-3), respectively) and their 1:1 mixture have been commonly used as oil industry "high-density brines." In our experiment they were added to tap
Preliminary experience with a modified premedication protocol that included intravenous diphenhydramine and calcium bromide for the prophylaxis of paclitaxel-related hypersensitivity reactions.
Saturated water solutions of calcium chloride, calcium bromide and their 1:1 mixture are commonly used as "high density brines" for pressure control in oil wells. To compare the effect of these chemicals of technical grade with the effect of the
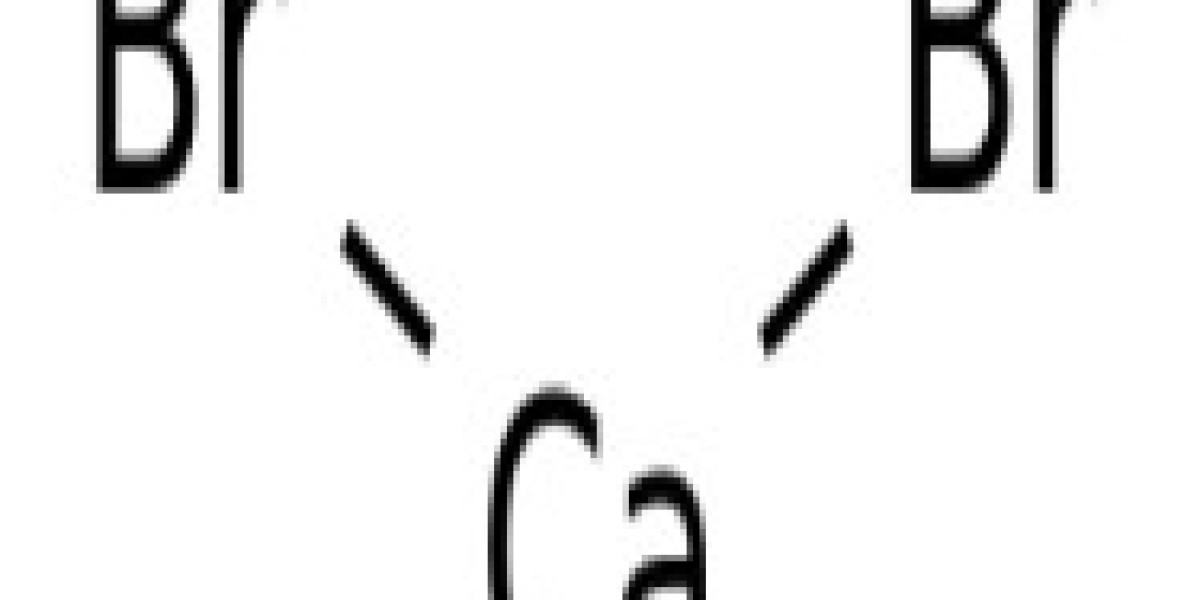
Calcium Bromide is a kind of white to off-white beads with a chemical formula of CaBr2 and a molecular weight of 199.89. Stanford Advanced Materials (SAM) offers high-quality Calcium Bromide (CaBr2) with competitive pricing.
Calcium Bromide is a kind of white to off-white beads. It is deliquescent and should be sealed and protected from light. It is used in the photography industry and also used as medicine.
Our Calcium Bromide (CaBr2) is carefully handled during storage and transportation to preserve the quality of our product in its original condition.
Calcium bromide is used in two main applications – clear brine fluids in oil & gas and as an oxidizer for mercury emissions control. In upstream oil and gas, calcium bromide is used to control wellbore pressures during completion and workover operations. In mercury emissions control, calcium bromide is sprayed on the coal prior to combustion and when the coal is burned the calcium bromide breaks down and reacts with elemental mercury to form ionic species of mercury that can be captured by downstream pollution control equipment.
An improved method for producing calcium bromide by reacting hydrogen bromide with calcium hydroxide in the presence of water and carbonate ions is disclosed. The improvement comprises maintaining a sufficient pH in the reaction mixture to convert at least a portion of the carbonate ions to a gaseous carbon-oxygen compound thereby removing the carbonate ions from the reaction mixture.
Search
Popular Posts
-
 Principle the Digi World - 6 Easy Methods to Understand Digital Marketing on Possess
By Clark Kent
Principle the Digi World - 6 Easy Methods to Understand Digital Marketing on Possess
By Clark Kent -
 ChatGPT Français - Utilisez ChatGPT gratuitement en français!
ChatGPT Français - Utilisez ChatGPT gratuitement en français!
-
 NSP Lecithin-Ergänzungen in Deutschland
By Tatiana
NSP Lecithin-Ergänzungen in Deutschland
By Tatiana -
 Heavenly Guidance: A Journey Through A Course in Miracles
Heavenly Guidance: A Journey Through A Course in Miracles
-
 Shining Souls (Trust): Pioneering Holistic Development and Recognized as the Best NGO in India
Shining Souls (Trust): Pioneering Holistic Development and Recognized as the Best NGO in India